The integrated rms velocity urms/u∞ (left) and Reynolds stress τ /u 2 ∞... | Download Scientific Diagram

Turbulent channel flow: the root-mean-square fluctuation velocity U +... | Download Scientific Diagram

Identify the correct labels of A, B and C in the following graph from the options given below:Root mean square speed urms; most probable speed ump; Average speed uav

Root-mean-square velocity profiles normalized by the friction velocity... | Download Scientific Diagram

What is the relationship between the average velocity (v) , root mean square velocity(u) and most probable velocity (alpha) :
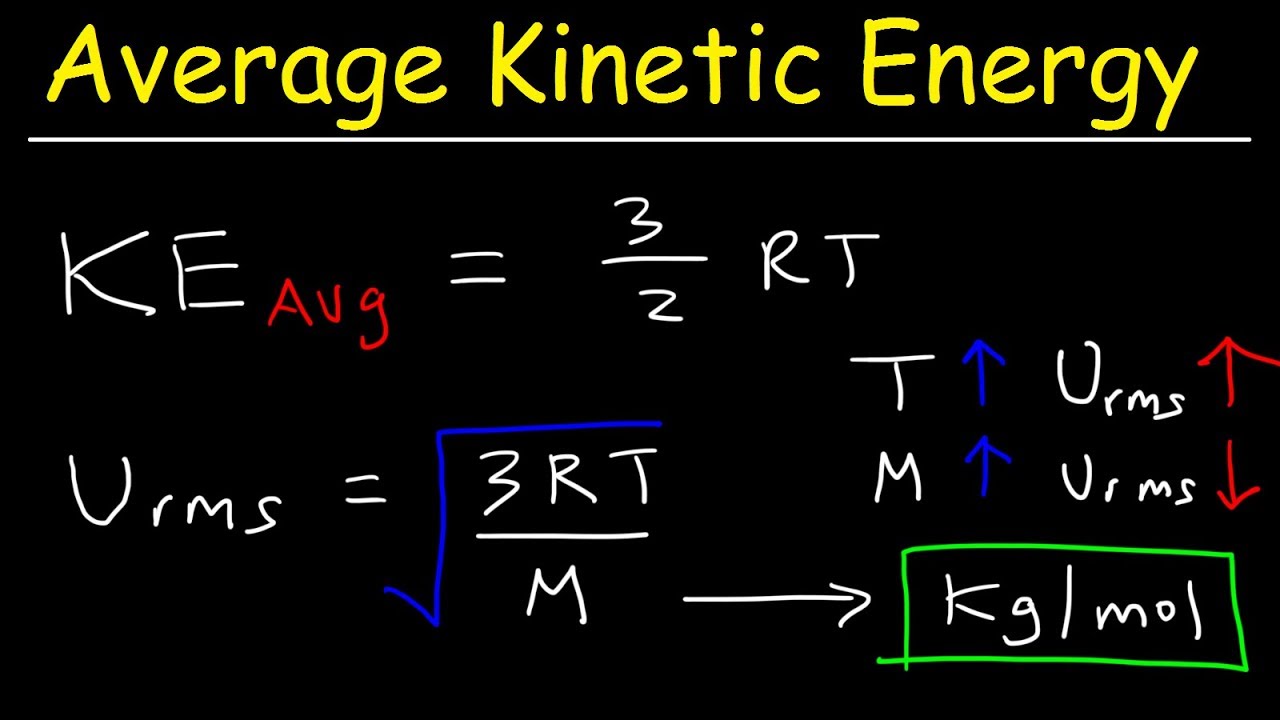
Average Kinetic Energy of a Gas and Root Mean Square Velocity Practice Problems - Chemistry Gas Laws - YouTube
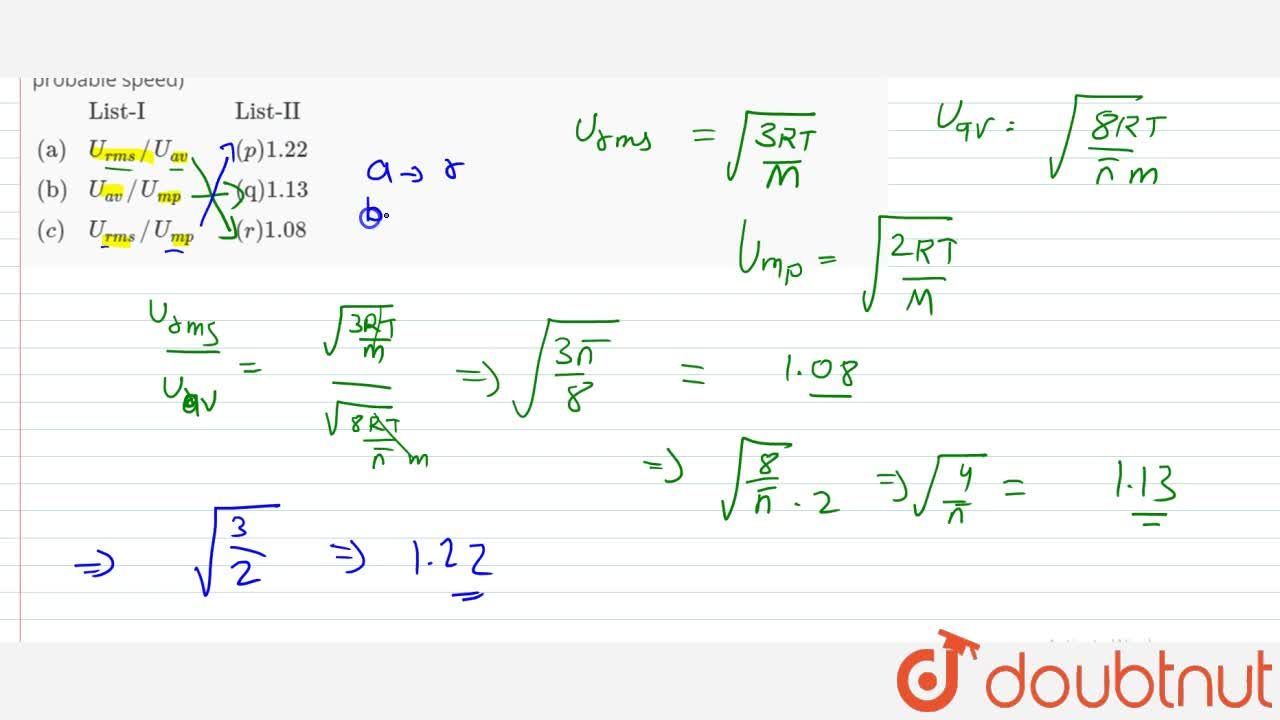
Match of following (where U(rms)=root mean square speed U(av) =average speed U(mp) = most probable speed) {:(,"List-I",,"List-II"),("(a)",U(rms)//U (av),,(p)1.22),("(b)",U(av)//U(mp),,"(q)1.13"),((c),U(rms)//U (mp),,"(r)1.08):}

Identify the correct labels of A, B and C in the following graph from the options given below:Root mean square speed urms; most probable speed ump; Average speed uav
Radial distributions of axial mean velocity (U) and rms velocity (Urms)... | Download Scientific Diagram
Which of the following statement(s) is(are) correct regarding the root mean square speed (Urms) and average translational - Sarthaks eConnect | Largest Online Education Community
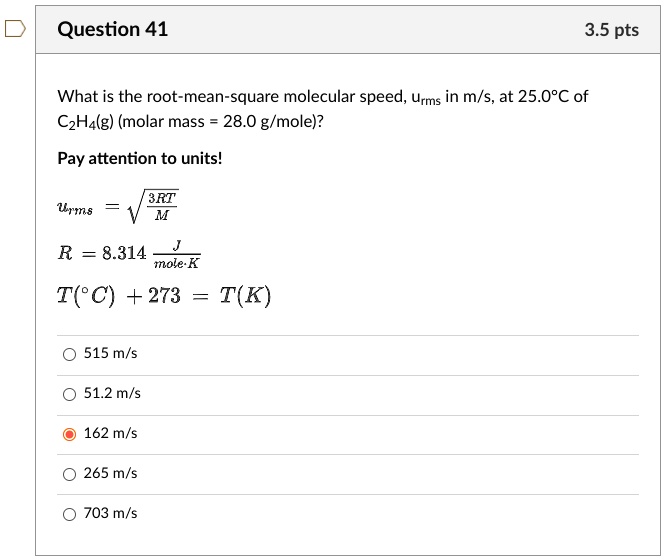
SOLVED: Question 41 3.5 pts What is the root-mean-square molecular speed, Urms in m/s, at 25.0'C of CzHalg) (molar mass 28.0 g/mole)? Pay attention to units! R 8.314 mole K T(C) +
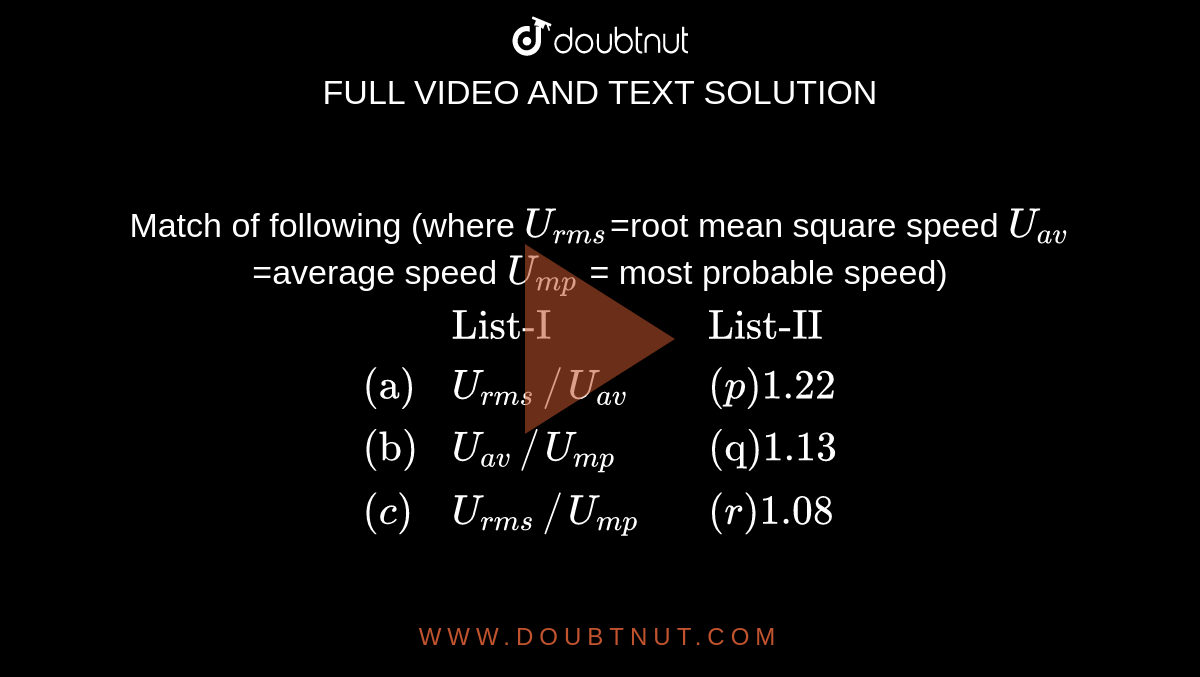
Match of following (where U(rms)=root mean square speed U(av) =average speed U(mp) = most probable speed) {:(,"List-I",,"List-II"),("(a)",U(rms)//U (av),,(p)1.22),("(b)",U(av)//U(mp),,"(q)1.13"),((c),U(rms)//U (mp),,"(r)1.08):}